Hello, welcome to Hangzhou Zhengda Medical Co., Ltd.
NEWS CENTER
PRODUCTS CLASS
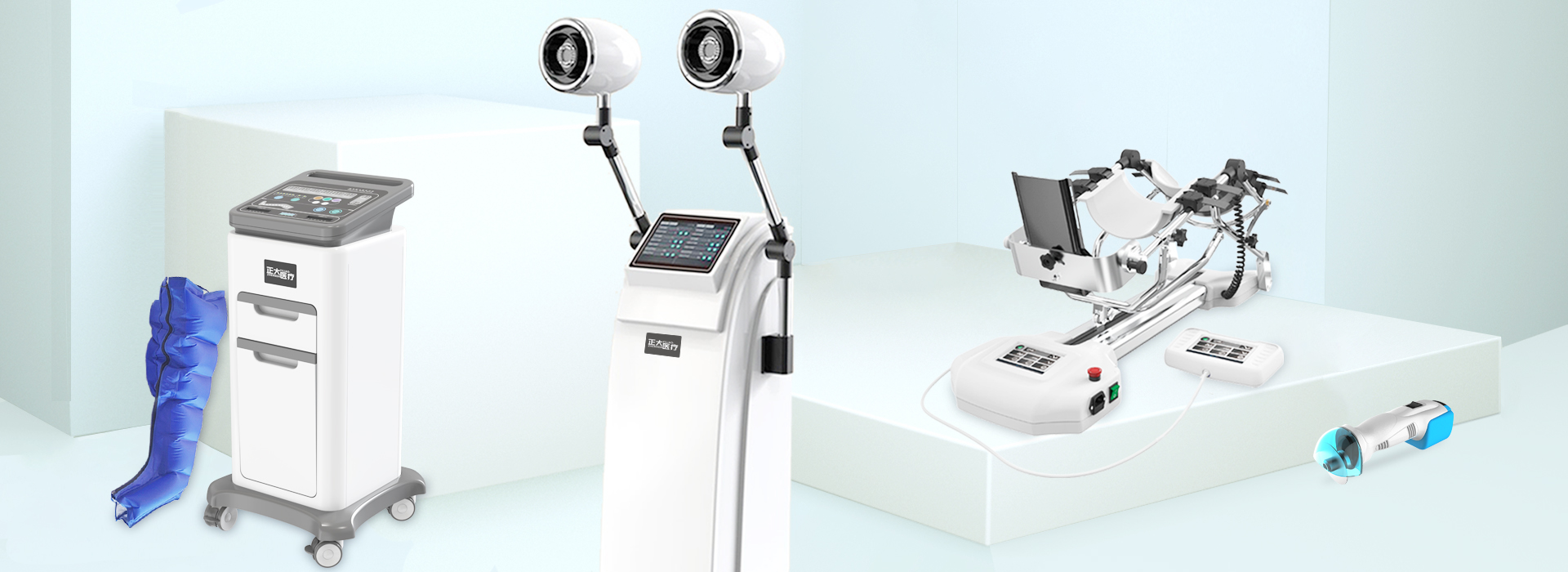
From October 28th to 31st, the 88th CMEF China International Medical Equipment Fair will be grandly opened at the Shenzhen International Convention and Exhibition Center (Baoan District). Our three manufacturing companies: Hangzhou Zhengda Medical Co., Ltd., Hunan Zhengda Medical Equipment Technology Co., Ltd. , Zhejiang Longyou Lande Medical Technology Co., Ltd., with joint rehabilitation device, electric pneumatic blood meter, pneumatic tourniquet, cervical and lumbar traction therapy device, pneumatic vibration expectoration machine, medical electric bone saw drill, air pressure wave therapy device, electric Equipment such as plaster saws, pneumatic finger training devices, shock wave therapy devices, cryotherapy devices, and fumigation therapy devices were on display.
We sincerely invite you to attend the exhibition. Warmly welcome colleagues, experts, new and old friends from all walks of life to visit the booth. Our company's booth number is 9C05.
The company was founded in 1991. It is an entity enterprise that develops and manufactures orthopedics, rehabilitation and operating room equipment. The enterprise has formed a group development model. Including Zhejiang Longyou Lande Medical Technology Co., Ltd., Hunan Zhengda Medical Technology Co., Ltd., etc. And established a physical manufacturing medical device company in Europe. The main R&D products have exceeded more than 20 series, basically orthopedic surgery equipment and physical rehabilitation equipment.
The company has strong full-process R&D capabilities. Including all processes including appearance design, structural design, mechanical parts design, electronic software and hardware design, logical interface design, etc. The company established the ISO13485 quality management system in 2005. And obtained multiple CE and FDA product registrations.
The company sincerely hopes to establish extensive and long-term cooperative relationships with experts and friends in the medical field and medical equipment industry! Welcome friends to visit and contact us.